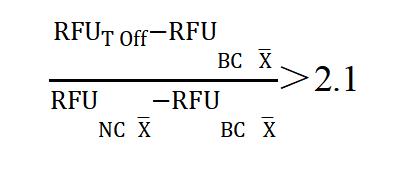- Preparation before experiment
Upon receipt of the shipment, please first centrifuge to concentrate the lyophilized powder to the bottom of the bottle, followed by carefully opening the cap. Dissolve the lyophilized powder in sterile water and ensure adequate mixing by shaking. Transform the solubilized plasmid into receptor cells for bulk amplification. Note that this plasmid is ampicillin-resistant, please use ampicillin-containing medium for screening when transforming into E. coli.
- Prepare Your Cell
According to the purpose of the experiment, select the appropriate cell line (please choose the highly efficient transfected cell line, 293T cells are recommended).
Use opaque black 96-well cell plates to culture the corresponding cells. Cells should be cultured in a constant temperature incubator until 80% confluence before proceeding to the subsequent transfection steps. Make sure the cells are evenly distributed to ensure the transfection efficiency and consistency of the experimental results.
- Prepare The TestSample
Take an appropriate amount of the test inhibitor sample to be determined, dissolve the sample using DMSO* or other appropriate solvent, and prepare a solution to the appropriate concentration using culture medium, configuring an appropriate concentration gradient for use if necessary. A commercialized protease inhibitor of the corresponding virus is recommended to be involved in the preparation as a control.
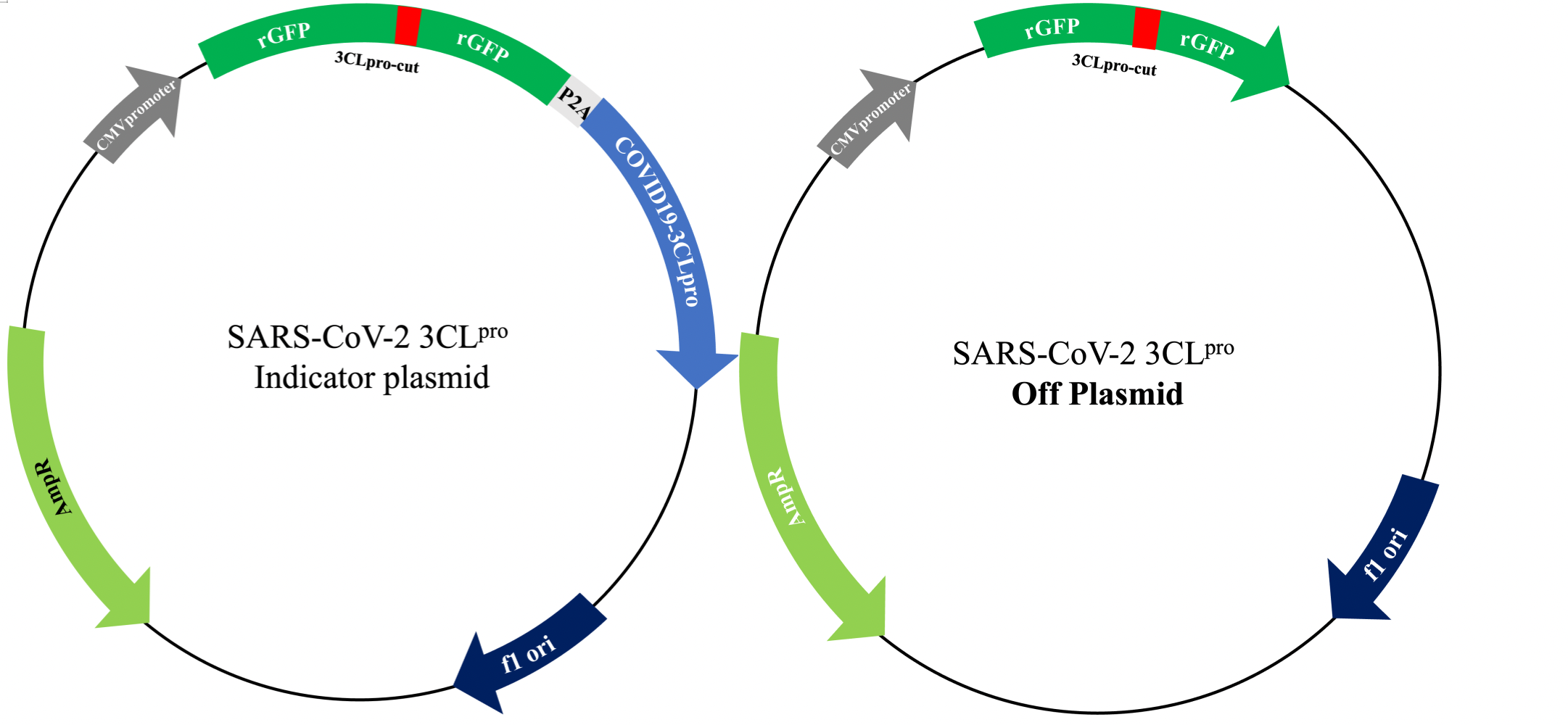
- 3CLproindicator plasmid and 3CLproOff plasmid transfection
a. Plasmid Transfection: 3CLproindicator plasmid and 3CLpro Off plasmid is transfected with 200ng/well respectively, and cultured in 5% CO2cell culture incubator at 37℃ for 4~6h.
b.Preparing Test Compound: Discard the cell culture supernatant at the time of transfection, and add 100μL/well of the diluted test sample. Cultivate in cell incubator at 37°C for 48 h. For more reliable results, it is recommended to perform at least 3 replicate wells for each dilution of the test compound.
|
Blank control, BC |
Negative Control, NC |
3CLpro Off plasmid |
Test Sample |
| Plasmid Transfection |
3CLpro indicator plasmid |
|
✅ |
|
✅ |
| 3CLpro Off plasmid |
|
|
✅ |
|
| Test Compound Preparation |
culture medium |
✅ |
✅ |
✅ |
✅ |
| Solvent* |
|
✅ |
✅ |
|
| Test Inhibitor |
|
|
|
✅ |
* If DMSO is used as a solvent, its final concentration in the culture wells needs to be controlled not to exceed 1% to avoid cell damage.
- Result Analysis
Our system provides two different ways for observing the results, please choose the appropriate method according to the purpose and stage of the experiment:
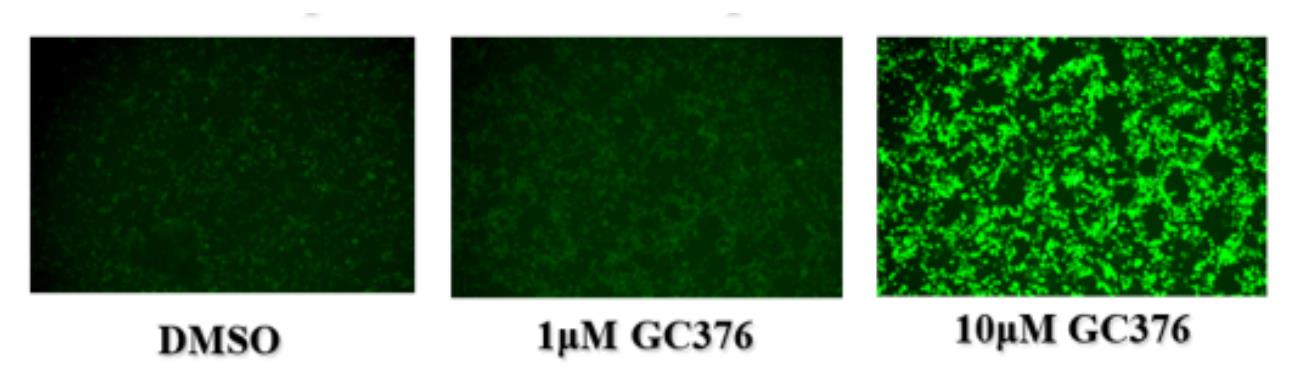
5.1 Qualitative analysis: For pre-screening of small amounts of drugs, the fluorescence intensity can be observed by fluorescence microscopy. This method is suitable for rapid qualitative analysis for initial estimation of the efficacy of inhibitors.
5.2 Quantitative analysis: If you want to determine the inhibition rate of the inhibitor or to calculate the IC50, a quantitative analysis is required, and the fluorescence value should be measured using a Fluorescence Microplate Reader, which is analyzed as follows:
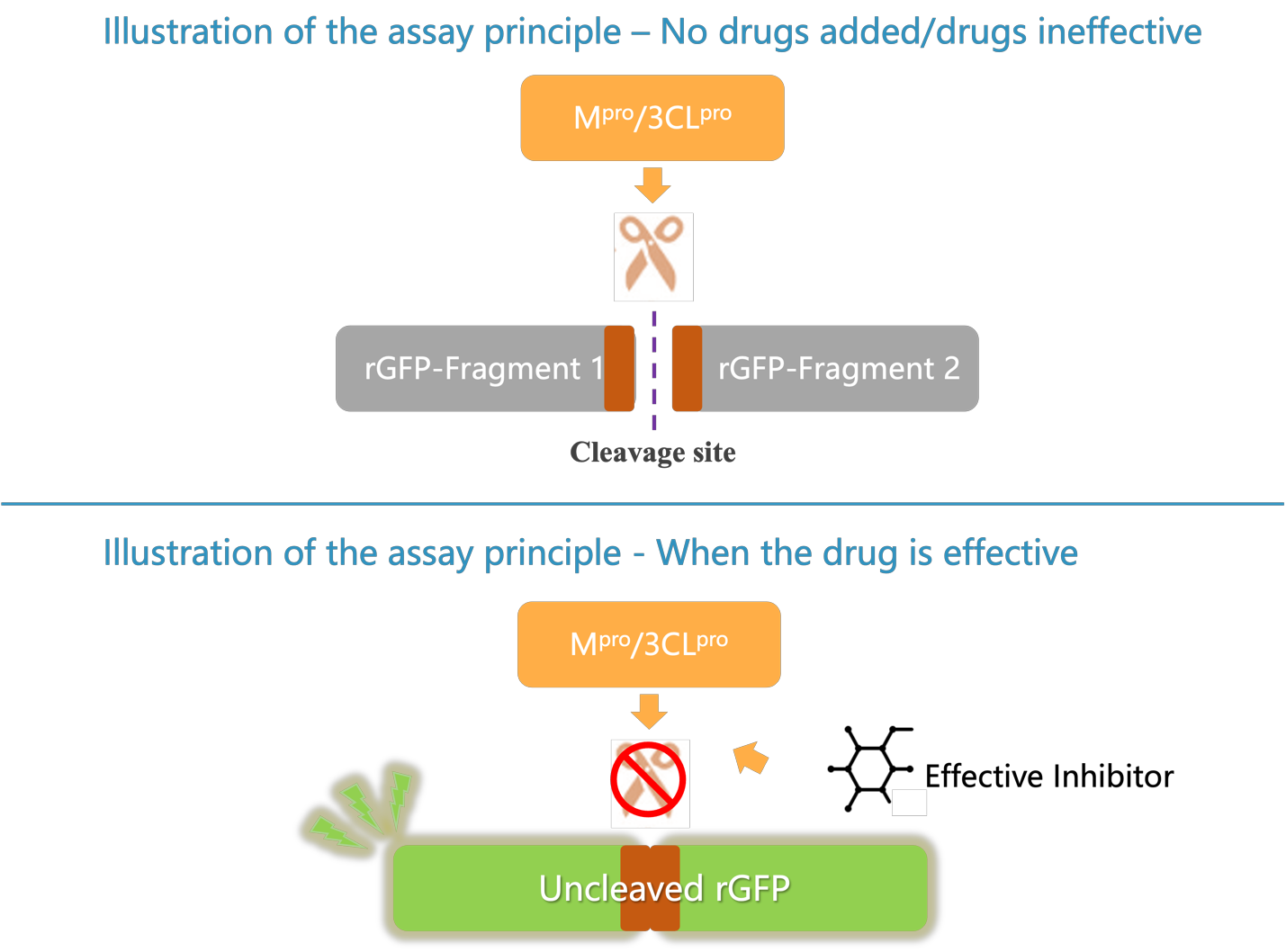
- Fluorescence Value: The temperature of the fluorescence zymograph is set to 37ºC; the excitation wavelength is set to 485 nm and the emission wavelength is set to 520 nm. Read and record the average fluorescence unit (RFU) per well as RFUNC, RFUT Offand RFUsample.
- Validity Criteria
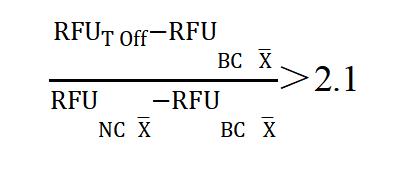
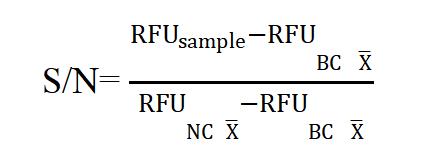
*The S/N is intended as a diagnostic response to differentiate positive from negative specimens.
| Positive |
S/N>1.5 |
| Negative |
S/N≤1.5 |
| Drug Concentration(μM) |
6.25 |
3.125 |
1.56 |
0.78 |
0.39 |
0.19 |
| Positive Well No. |
3 |
3 |
3 |
0 |
0 |
0 |
| Negative Well No. |
0 |
0 |
0 |
3 |
3 |
3 |
| Positive Rate |
100% |
100% |
100% |
0% |
0% |
0% |
Distance Proportion=(Positive Rate>50%-50%)/(Positive Rate>50%-Positive Rate<50%)
=(100%-50%)/(100%-0%)
=0.5
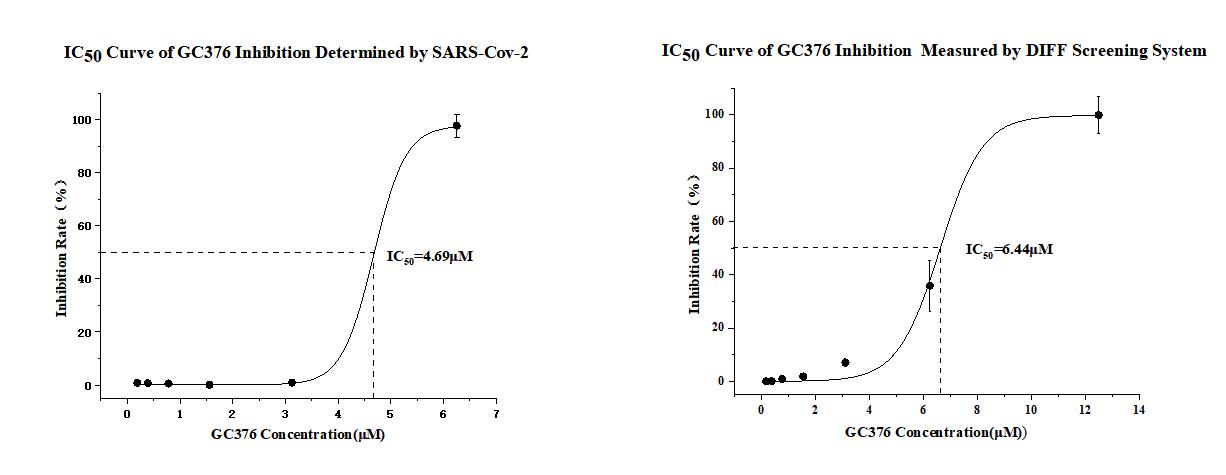
Log10(IC50) = Log10 (Drug Concentration>50%)- Distance Proportion*Log10(Dilution Interval*)
= Log10 (1.56)-0.5*Log10(2)
=0.19-0.5*0.3
=0.04
IC50=100.04=1.1μM
* Dilution Interval refers to the fixed ratio used in each step of a series of dilutions in an experiment. In the sample calculation above, we used a dilution interval of 2.
General Considerations
- Product inspection: Before use, takeout the assaysystem from the product box and verify that all components are intact and undamaged.
- Before first use, it is recommended to centrifuge the reagents briefly (a few seconds) to concentrate the liquid at the bottom of the tube.
- In the case of frozen reagents, it is important to ensure that they are completely thawed and homogeneously mixed before use. Avoid repeated freezing and thawing to prevent plasmid degradation.
- It is recommended to use an opaque 96-well black plate for the assay to minimize optical interference when reading the values.
- In general, a higher fluorescence value indicates a stronger inhibition of the corresponding viral protease in the sample to be tested. However, if the drug is cytotoxic and causes cell damage, the fluorescence value may decrease accordingly. Therefore, it is recommended that the cytotoxicity of the drug be evaluated and a safe dose range determined prior to the experiment.
- The intensity of the fluorescence value may be affected by different cell lines or transfection efficiency, and the calculated IC50result can provide an effective reference for scientific research.
- This assay system will perform optimally for up to 12 months from date of receipt when the materials are stored as directed.