“DIFF+” against influenza
01 Preface
From 1918 to now, human beings have experienced more than 100 years of struggle with influenza. According to the Global Influenza Strategy Report 2019-2030 published by the World Health Organization (WHO), in an outbreak pandemic, influenza can spread rapidly around the world and can affect 10-20% of the total population. Even in non-outbreak pandemic years, seasonal influenza causes about 3-5 million severe cases and 290,000-650,000 deaths globally each year.
To fight the influenza virus, we need to rely on oral medications and vaccines to work together. Currently, China is in the midst of an influenza epidemic. According to data, the sales of influenza drugs in China have been rising in the past three years, and the sales of influenza drugs in China in the first half of 2023 amounted to about 3.682 billion yuan. Influenza cases are still surging every year, which is expected to drive the continuous growth of the anti-influenza drug market. Influenza vaccine is a heavyweight variety in the global vaccine industry, with huge market space, while the domestic influenza vaccine penetration rate is low, and there is still a huge gap with developed countries. As of 2020, the total number of domestic influenza vaccines approved and issued is 57.65 million doses, according to the end of 2020, the total population of 1,412,120,000 people, even if the vaccine issued that year to realize all the vaccination, the vaccination rate of the whole population is only 4.08%.
According to the mechanism of action, anti-influenza virus drugs are mainly classified into three categories: neuraminidase inhibitors (oseltamivir, paramivir, zanamivir), hemagglutinin inhibitors (abidol) and RNA polymerase inhibitors (marbofloxacavir, favipiravir).
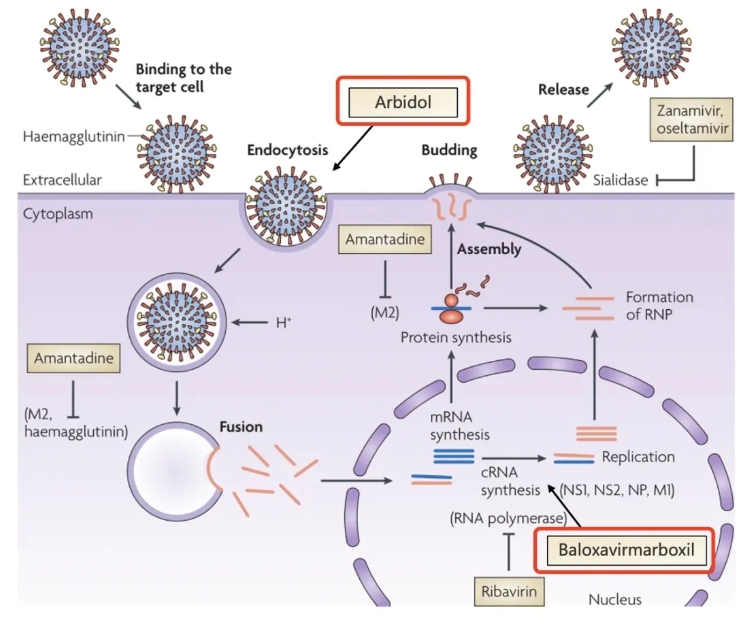
Antiviral drug development targets and representative drugs source: Mark von Itzstein, Nature Reviews Drug Discovery, 2007
Challenges in antiviral drug development
The therapeutic time window for currently used anti-influenza drugs is relatively narrow. For example, oseltamivir must be initiated within 48 hours of influenza virus infection to achieve optimal efficacy. This time constraint poses a challenge to the development of new drugs that not only need to rapidly and effectively suppress the virus at its peak of replication, but also show efficacy later in the course of the disease to reach patients who have missed the initial therapeutic window. Therefore, researchers need to find or design drugs that are effective and safe over a wider therapeutic window, which adds to the complexity and cost of drug development.
At the same time, the susceptibility of influenza viruses to drug-resistant mutations also poses a challenge for new drug development. Resistance to neuraminidase inhibitors, such as oseltamivir and zanamivir, is increasing due to mass use or misuse of these drugs, and resistance to baloxavir, a nucleic acid endonuclease inhibitor, is rapidly developing in phase III clinical trials. There is currently no effective solution for influenza virus resistance.
In addition, existing marketed drugs have relatively limited therapeutic effects on patients with severe influenza. Therefore, there is an urgent need to develop new anti-influenza viruses to meet the urgent need for clinical treatment of influenza patients.
“DIFF+” Anti-Influenza
02 DIFF Rense Bio’s preclinical evaluation platform for antiviral drugs – Screening and efficacy evaluation
DIFF Rense Bio’s preclinical CRO platform covers ex vivo and in vivo efficacy evaluation of different types of influenza viruses. It is capable of performing Minigenome RNP (polymerase activity assay) and utilizing random mutation virus library technology to predict drug resistance sites in advance, which can point out the direction for drug discovery and development.
“DIFF+ Anti-Influenza
03 DIFF R&S Vaccine Development Service Platform – Safety, Stability, Efficacy Evaluation & Virus Strain Development
The influenza vaccine development service of DIFF R&S Biologicals covers the in vitro and ex vivo safety evaluation of different types of influenza vaccine candidates, as well as the evaluation of vaccine immunogenicity and virulence protection efficiency, and other integrated services.
“DIFF+” Anti-influenza
04 Service Core Advantages
1. Advantage of Bio Database strain resources:
2. Advantage of Tech Database technology resources
DIFF R&S Bio has a mature reverse genetic manipulation technology platform with industry-leading technological advantages:
Influenza Virus Reporting Virus System:
By using viral reverse genetic manipulation technology, Defruns inserts reporter genes into the influenza virus genome. This advanced technology provides researchers with an efficient, intuitive and reliable tool. Influenza reporter viruses allow us to efficiently and intuitively observe the dynamic infection process of the virus in ex vivo and in vivo experiments. The expression level of reporter proteins can be used to quantitatively analyze the replication of the virus and to evaluate the efficacy of antiviral drugs.
Polymerase activity assay (Minigenome RNP):
The polymerase activity assay allows us to reconstruct the gene replication and transcription process of influenza virus in the laboratory, thus evaluating the activity and function of influenza virus polymerase. Playing an important role in the screening of antiviral drugs, researchers can utilize this technology to assess the effects of different antiviral drugs on influenza virus polymerase activity, thus providing an important reference for drug development.
Construction of resistant strains Prediction of resistance sites (Random mutation virus library technology):
DEFRANCE has an advanced technology for constructing drug-resistant strains and predicting drug-resistant sites – Randomized Mutant Virus Library (RMVL) technology. The core of this technology is the introduction of random mutations to generate a large-scale library of viral variants, which can then be screened or sequenced to identify drug-resistant mutants. Through random mutation virus library technology, we can generate a large number of viral variants, which can effectively study the mechanism of drug resistance and provide important reference and guidance for the development of antiviral drugs.
Defective Interfering Influenza Virus Particles (DIIVPs):
In the study of highly pathogenic avian influenza viruses, the direct use of intact viruses for experiments may pose a safety risk due to their high degree of pathogenicity and transmissibility, making the use of DIIVPs an alternative approach. DIIVPs mitigate the risks involved in experimental manipulation by constructing defective viruses through the deletion of viral pathogenic genes via a proven reverse genetic platform, which prevents the virus from fully replicating after infecting host cells.DIIVPs provide a safe and effective research tool for studying highly pathogenic avian influenza viruses and can be used to evaluate the efficacy of vaccines and antivirals at the cellular level, offering scientists the opportunity to conduct influenza virus-related research in the ordinary environment for influenza virus-related research.
VSV-Flu Pseudovirus (VSV-FLU):
Vesicular Stomatitis Virus (VSV) vector of influenza pseudovirus is another tool used to study influenza viruses. It is constructed by replacing the capsid protein of VSV with the capsid protein of influenza virus, thus turning VSV into a pseudovirus capable of carrying the influenza virus genome. By using a combination of VSV-FLU pseudoviruses and DIIVPs pseudoviruses, researchers can comprehensively evaluate various targets of highly pathogenic avian influenza. influenza pseudoviruses with VSV vectors are a safe and highly efficient tool that provides important support for the development of novel vaccines and antiviral drugs.
“DIFF+” against influenza
05 Case Sharing
1. Reporter virus
Fluorescent reporter virus – cellular level
The influenza fluorescent reporter virus constructed by Diff can produce strong fluorescent signals after infecting cells, indicating that the virus can replicate and express GFP normally in cells;
The influenza fluorescent reporter virus constructed by Diff can form empty spots, which has the same function as WT virus.
2. Mouse Adaptations – Evaluation of Efficacy
Mice are now widely used to study pathogenesis, viral replication, host immune responses, and the efficacy of antiviral drugs and vaccines. Mice infected with non-adapted human influenza viruses generally do not show typical human influenza symptoms, such as fever and respiratory symptoms (sneezing, runny nose, coughing, etc.); some highly pathogenic avian influenza viruses can cause disease in mice, and the clinical signs of influenza infection in mice include arching of the back, disheveled coat, loss of appetite, weight loss, neurological symptoms (paralysis of the hind limbs) and death.


